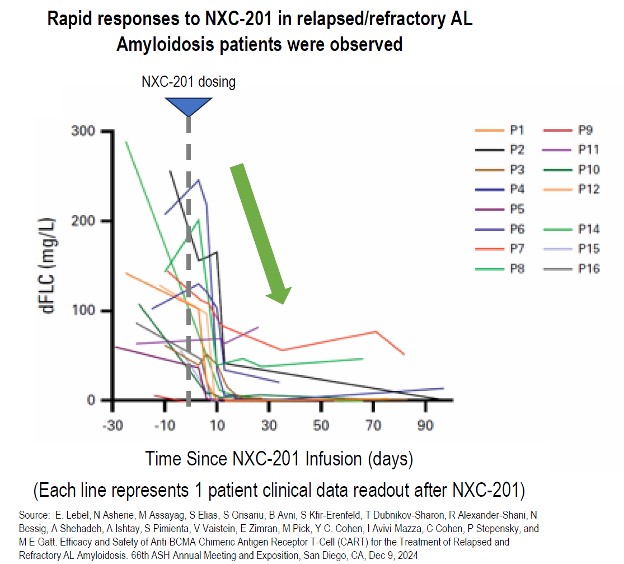
75% (12/16) complete response (CR) rate observed in standard of care (Dara-CyBorD) relapsed/refractory AL Amyloidosis patients with median 4 lines of prior therapy in updated Phase 1/2 data as of December 9, 2024 Best responder duration of response was 31.5 months with complete response ongoing as of December 9, 2024 Conference call to discuss results Tuesday, December 10, 2024 4:30 p.m.
ET at https://zoom.us/j/94736340854?pwd=LTBtu2LuvwSb6S6ISuH5yKTDLsI2vt.1 LOS ANGELES, CA, Dec.
10, 2024 (GLOBE NEWSWIRE) -- Immix Biopharma, Inc. (“ImmixBio”, “Company”, “We” or “Us” or ”IMMX”), a clinical-stage biopharmaceutical company developing cell therapies for AL Amyloidosis and select immune-mediated diseases, today announced that new NXC-201 NEXICART-1 clinical data in relapsed/refractory AL Amyloidosis has been presented at 66th American Society of Hematology (ASH) Annual Meeting being held in San Diego, California. The updated results include follow-up and clinical data from 3 new NEXICART-1 patients.
“We are encouraged by the updated NXC-201 results being presented by our academic collaborators at ASH 2024. We believe the high percentage of complete responders, combined with the consistent, attractive tolerability profile is critically important in relapsed/refractory AL Amyloidosis. This expanded NXC-201 dataset continues to bolster our leadership in relapsed/refractory AL Amyloidosis, where no drugs are FDA approved today,” said Ilya Rachman, M.
D., Ph.D.
, Chief Executive Officer of Immix Biopharma. Gabriel Morris, Chief Financial Officer of Immix Biopharma, added, “We are looking forward to bringing this promising therapy to U.S.
relapsed/refractory AL Amyloidosis patients.” Immix Biopharma Announces 75% Complete Response Rate (n=16); 31.5 months Best Response Duration (ongoing) for CAR-T NXC-201 in Relapsed/Refractory AL Amyloidosis Patients at ASH 2024 Immix Biopharma, Inc.
(Nasdaq:IMMX) At the NXC-201 ASH 2024 oral presentation, data were presented from 16 relapsed/refractory AL amyloidosis patients (including 3 new patients) in the ongoing Phase 1b/2 NEXICART-1 study, with median 4 lines of therapy prior to NXC-201. Patients were infused with CAR+T cells at doses of 150 x 10 6 (n=1), 450 x 10 6 (n=2), and 800 x 10 6 (n=13). Patient characteristics: 81% (13/16) had cardiac involvement 38% (6/16) had New York Heart Association (NYHA) stage 3 or 4 heart failure (3 stage 4, 3 stage 3) 31% (5/16) had Mayo stage 3 (1 stage 3b, 4 stage 3a) AL amyloidosis disease 44% (7/16) had t(11;14) translocation Relapsed/refractory to a median 4 lines of prior therapy (range: 3-10) Safety and efficacy data: Overall response rate of 94% (15/16) Complete response rate of 75% (12/16) (9 out of 16 were MRD- 10 -5 ) Organ response rate of 62% (8/13 evaluable) Best responder had a duration of response of 31.
5 months as of December 9, 2024, with complete response ongoing There were no immune effector cell-associated neurotoxicity syndrome (ICANS) events Median CRS duration was 2 days (range: 1-5): No grade 4 cytokine release syndrome (CRS) events 2 experienced no CRS; 3 experienced grade 1 CRS; 8 Experienced grade 2 CRS; 3 experienced grade 3 CRS The NXC-201 66 th American Society of Hematology Meeting oral presentation video can be accessed on the ASH website: https://annualmeeting.hematology.org/session/250954 .
The NXC-201 66 th American Society of Hematology Meeting oral presentation can be accessed on the ImmixBio corporate website at this link: https://www.immixbio.com/ .
ASH Presentation Details (CAR-T NXC-201 in relapsed/refractory AL Amyloidosis). Publication #894 Session Date: Monday, December 9, 2024 Session Name: 652. MGUS, Amyloidosis, and Other Non-Myeloma Plasma Cell Dyscrasias: Clinical and Epidemiological: Ignored no Longer-Progress in AL Amyloidosis Session Time: 2:45 PM-4:15 PM Presentation Time: 4:00PM PT About NEXICART-1 NEXICART-1 (NCT04720313) is an open-label, ex-U.
S. Phase 1b/2 clinical trial of NXC-201 (formerly HBI0101) in patients with relapsed/refractory multiple myeloma and relapsed/refractory AL amyloidosis (including AL Amyloidosis patients with impaired cardiac function and including AL Amyloidosis patients exposed to prior BCMA-targeted therapy). The primary objective of the study is to characterize the safety and efficacy of NXC-201.
NEXICART-1 clinical results are available at https://immixbio.com/the-science-pipeline-and-publications/ . About NEXICART-2 NEXICART-2 (NCT06097832) is an open-label, single-arm, multi-site U.
S. Phase 1b/2 dose expansion clinical trial of CAR-T NXC-201 in relapsed/refractory AL Amyloidosis. NEXICART-2 is expected to enroll 40 patients with adequate cardiac function who have not been exposed to prior BCMA-targeted therapy.
The study is designed with a standard 6 patient safety-run in to evaluate two doses (three patients each at 150 million CAR+T cells and 450 million CAR+T cells) (both dose levels were evaluated in the NEXICART-1 study and have produced complete responses in relapsed/refractory AL Amyloidosis patients). The study aims to evaluate the safety and efficacy of NXC-201. Primary endpoints are complete response rate and overall response rate, according to consensus recommendations (Palladini et al.
2012). About NXC-201 NXC-201 is a sterically-optimized BCMA-targeted chimeric antigen receptor T (CAR-T) cell therapy. Initial data from Phase 1b/2 ex-U.
S. study NEXICART-1 has demonstrated high complete response rates and no neurotoxicity of any kind in AL Amyloidosis. NXC-201 is being studied in a comprehensive clinical development program for the treatment of patients with relapsed/refractory AL amyloidosis in the U.
S., with the potential to expand into select immune-mediated diseases. The NXC-201 NEXICART-2 (NCT06097832) U.
S. clinical trial builds on a robust clinical dataset. NXC-201 has been awarded Orphan Drug Designation (ODD) in AL Amyloidosis by the US FDA and in the EU by the EMA.
About AL Amyloidosis AL amyloidosis is caused by abnormal plasma cells in the bone marrow, which produce misfolded amyloid proteins that build-up in the heart, kidney, liver, and other organs. This build-up causes progressive and widespread damage to multiple organs, including heart failure, and leads to high mortality rates. The U.
S. observed prevalence of relapsed/refractory AL Amyloidosis is estimated to be growing at 12% per year according to Staron, et al Blood Cancer Journal, to approximately 33,277 patients in 2024. The Amyloidosis market was $3.
6 billion in 2017, and is expected to reach $6 billion in 2025, according to Grand View Research. About Immix Biopharma, Inc. Immix Biopharma, Inc.
(ImmixBio) (Nasdaq: IMMX) is a clinical-stage biopharmaceutical company developing cell therapies for AL Amyloidosis and select immune-mediated diseases. Our lead candidate is sterically-optimized BCMA-targeted chimeric antigen receptor T (CAR-T) cell therapy NXC-201. NXC-201 is being evaluated in the U.
S. Phase 1b/2 trial NEXICART-2 (NCT06097832) as well as the ex-U.S.
study NEXICART-1 (NCT04720313). NXC-201 has demonstrated no neurotoxicity of any kind in AL Amyloidosis and short duration of cytokine release syndrome (CRS), supporting expansion into select immune-mediated diseases. NXC-201 has been awarded Orphan Drug Designation (ODD) in AL Amyloidosis by the US FDA and in the EU by the EMA.
Learn more at www.immixbio.com and www.
BeProactiveInAL.com . Forward Looking Statements This press release contains forward-looking statements regarding Immix Biopharma, Inc.
, its results of operations, prospects, future business plans and operations and the matters discussed above, including, but not limited to, the potential benefits of our product candidate CAR-T NXC-201 and the timing and results related clinical trials. These statements involve risks and uncertainties, and actual results may differ materially from any future results expressed or implied by the forward-looking statements. Forward-looking statements also include, but are not limited to, our plans, objectives, expectations and intentions and other statements that contain words such as “expects”, “contemplates”, “anticipates”, “plans”, “intends”, “believes”, “estimates”, “potential”, and variations of such words or similar expressions that convey the uncertainty of future events or outcomes, or that do not relate to historical matters.
Those forward-looking statements involve known and unknown risks, uncertainties and other factors that could cause actual results to differ materially. Among those factors are: (i) the risk that the further data from the ongoing Phase 1b/2 clinical trials for CAR-T NXC-201 will not be favorably consistent with the data readouts to date, (ii) the risk that the Company may not be able to commence the NEXICART-2 multi-site U.S.
Phase 1b/2 clinical trial; (iii) the risk that the Company may not be able to advance to registration-enabling studies for CAR-T NXC-201 or other product candidates, (iv) that success in early phases of pre-clinical and clinicals trials do not ensure later clinical trials will be successful; (v) that no drug product developed by the Company has received FDA pre-market approval or otherwise been incorporated into a commercial drug product, (vi) the risk that the Company may not be able to obtain additional working capital with which to continue the clinical trials for CAR-T NXC-201, or advance to the initiation of registration-enabling studies, for such product candidates as and when needed and (vii) those other risks disclosed in the section “Risk Factors” included in the Company’s Annual Report on Form 10-K filed with the SEC on March 29, 2024 and other periodic reports subsequently filed with the Securities and Exchange Commission. These reports are available at www.sec.
gov. Immix Biopharma cautions that the foregoing list of important factors is not complete. Immix Biopharma cautions readers not to place undue reliance on any forward-looking statements.
Immix Biopharma does not undertake, and specifically disclaims, any obligation to update or revise such statements to reflect new circumstances or unanticipated events as they occur, except as required by law. If we update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements. Contacts Mike Moyer LifeSci Advisors mmoyer@lifesciadvisors.
com Company Contact [email protected] Attachment Immix Biopharma Announces 75% Complete Response Rate (n=16); 31.5 months Best Response Duration (ongoing) for CAR-T NXC-201 in Relapsed/Refractory AL Amyloidosis Patients at ASH 2024.












/s3/static.nrc.nl/images/gn4/stripped/data125457065-964e6e.jpg)